

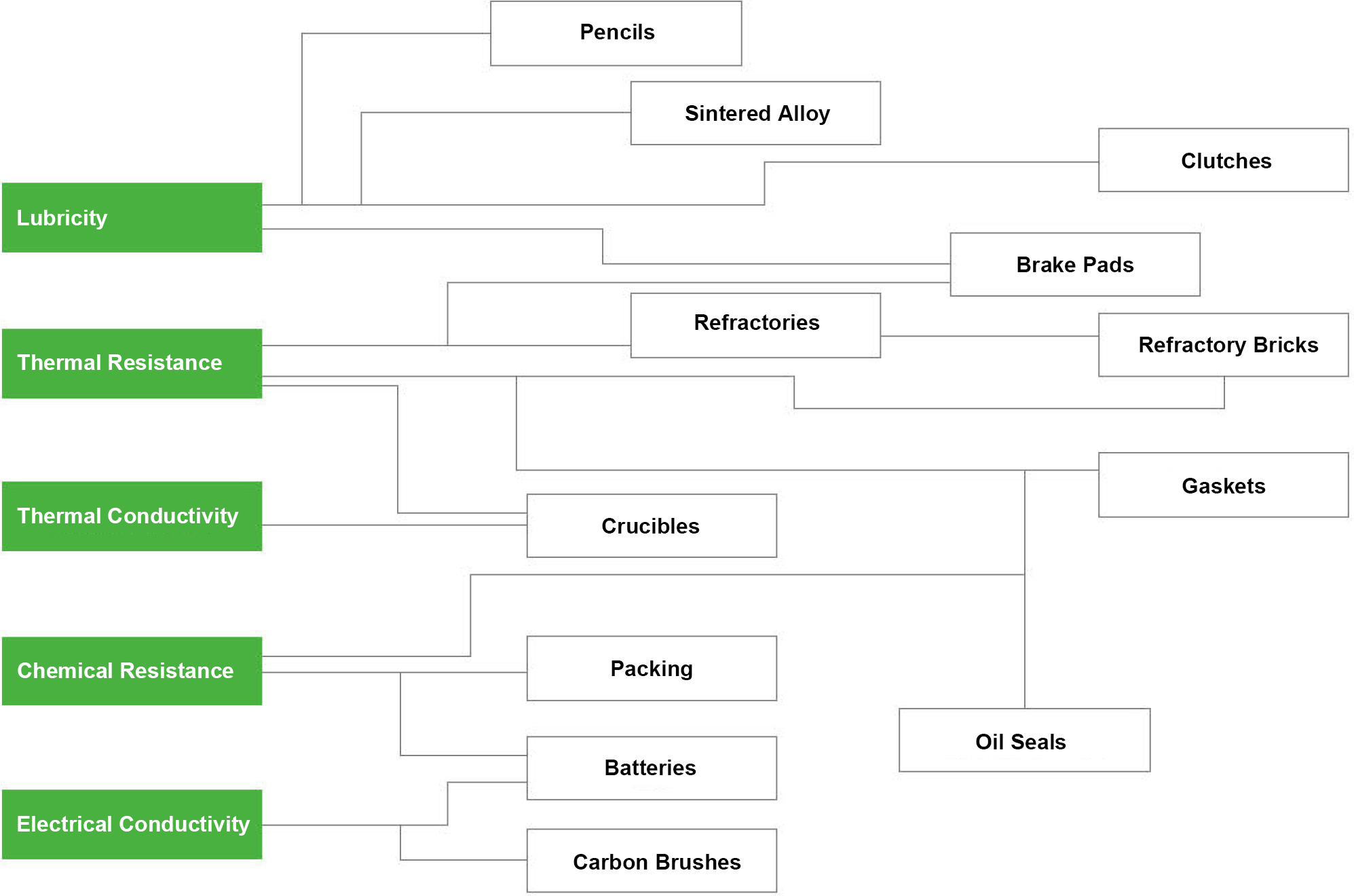
Graphite has many excellent properties, and the following five are representative of these properties. Each of these properties is used in various applications such as lubricants, refractories, and electrical materials.

Lubricity
Graphite has also high lubricity in the crystalline state. Especially the performance does not change much even in a high temperature atmosphere or in a place with a high load, therefore it can also be used for maintenance-free lubrication methods.

Chemical Resistance
Graphite is very stable because it has covalent crystals of carbon. As a result, it is stable against both acid and alkali chemicals and it shows high chemical resistance.

Thermal Resistance
It can withstand extreme low to high temperatures of -200°C to 450°C in the atmosphere and 3000°C in a non-oxidizing atmosphere. Graphite is resistant to rapid heat changes, and its strength increases at high temperatures (up to 2500°C). The strength is increased up to about 2 times.

Thermal Conductivity
The thermal conductivity of graphite is a good conductor of heat comparable to metals. Due to the free electrons of the graphite crystal, it exhibits high thermal conductivity especially to the direction of the crystal plane.
Electrical Conductivity
The electrical resistance of graphite, which value is 1375.0 × 10-6Ωcm, is slightly inferior to the metals of copper, silver, and gold, etc. However, it is less likely to be oxidized than copper and less expensive than silver and gold, therefore it has been used in wide range.
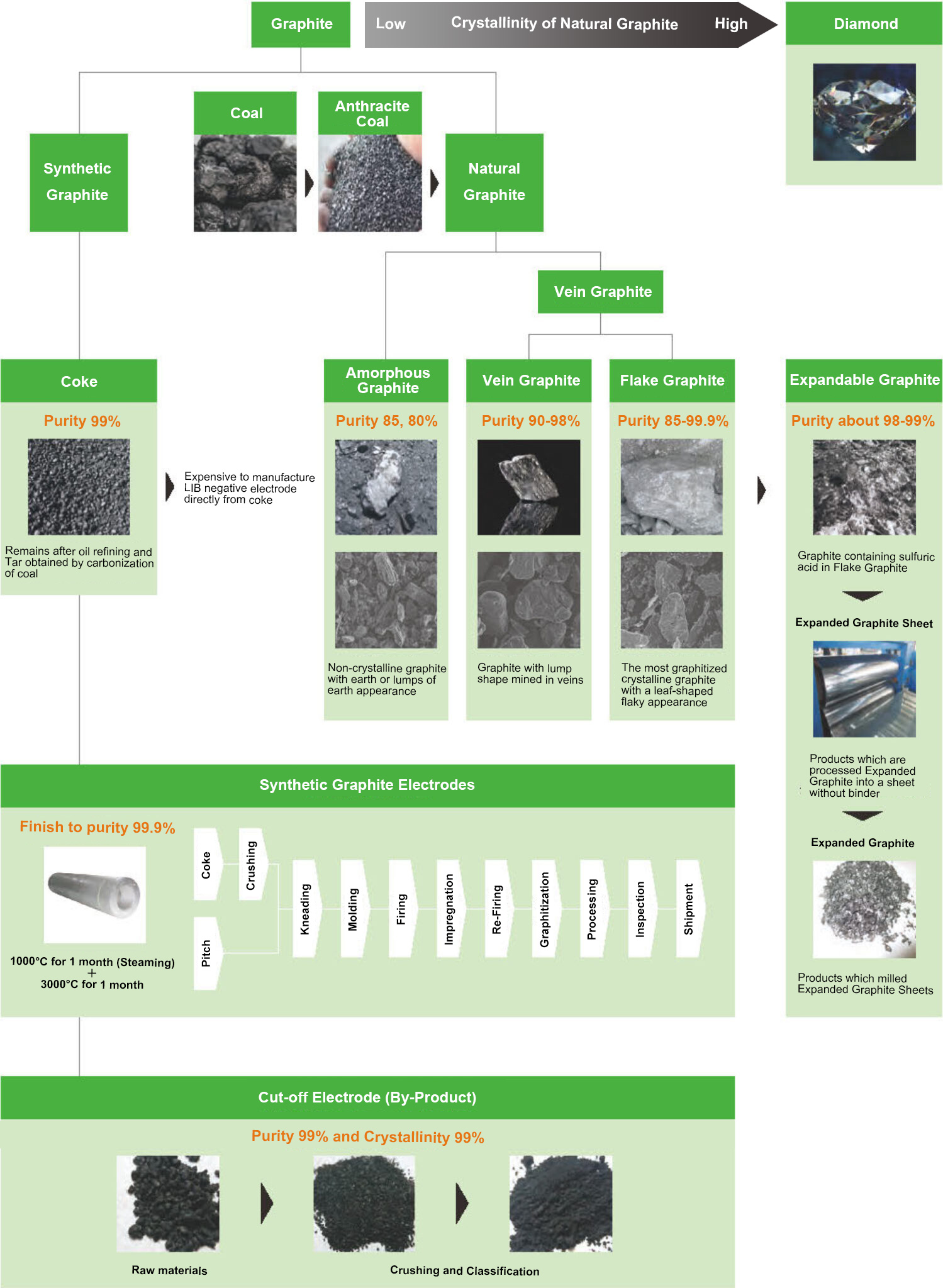
Graphite can be easily categorized as shown below.
If you have any questions or concerns, please feel free to contact us.